





High-intensity focused ultrasound ablation: a new strategy to manage primary bone tumors
Wenzhi Chen and Kun Zhou
Purpose of review
This review attempts to summarize the current status of the experimental and clinical studies using high intensity focused ultrasound ablation to manage malignant primary bone tumors.
Recent findings
A novel extracorporeal and noninvasive strategy for managing solid tumors compared with surgical removal or other minimally invasive modalities, high intensity focused ultrasound ablation has been used mostly in
Summary
Although further investigations on long-term follow-up results after high intensity focused ultrasound ablation are still needed, this modality may be one of the most promising treatment technologies for bone tumors and limb salvage in the future.
Keywords
bone tumor, high intensity focused ultrasound, osteosarcoma, treatment
Current Opinion in Orthopaedics 2005, 16:494—500
Abbreviations
CDFI color Doppler flow imaging
DSA digital subtraction angiography
HIFU high-intensity focused ultrasound
SARR specific absorption rate ratio
Introduction
This review discusses the theory, animal experiments, and clinical results using high intensity focused ultrasound (HIFU) ablation for managing primary bone tumors. Some issues of most concern such as outcome, imaging assessment, and immune responses after the therapy are discussed. By summarizing the present situations of HIFU ablation, this review intends to provide some ideas for further studies.
Surgical removal and radiation therapy are commonly used strategies to treat bone tumors. The former is often used for primary bone tumors, whereas the latter is more often than not adopted for bone metastases. After surgical removal of primary bone tumors, the limb reconstruction could be done using the removed tumor-bearing bone section after extracorporeal tumor inactivation. Some physicaland chemical methods are used to inactivate tumorbearing bone sections, including hyperthermia and high pressure [1,2], hypothermal processing [3], high-dose radiation therapy [4], and alcohol inactivation and replantation [5]. For the past 20 years, some surgeons exposed tumor lesions to microwave after surgical procedure and had the tumor cells killed in situ, and the inactivated tumor-bearing bone sections were used for limb reconstruction.This technique is beneficial to adhesion of ligaments and tendons [6,7]. Percutaneous radio frequency for osteoid osteoma was a surprising start for minimally invasive treatment of bone tumors [8•,9•]. Although intraoperative hyperthermia has been considered as an efficient therapy for managing bone tumors, the surgical procedures are complex and appear to be an invasive or minimally invasive method. High intensity focused ultrasound is a new attractive extracorporeal method of noninvasive tissue ablation.High intensity ultrasound beams can be introduced to target sites lying deep within the patient’s body. If sufficient sound energy is concentrated within focal volume, temperature at a specific location may be raised to 56–80_C in a few seconds [10]. As a result, a welldelineated coagulative necrosis can be obtained in the target tissue, whereas surrounding normal tissues remain almost undamaged.
The targeted tissue necrosis produced using HIFU was first reported in the 1940s [11]. Fry et al. [12] successfully destroyed small regions in the human brain without damaging surrounding tissues by HIFU in the 1950s. Since then, a number of reports indicated that HIFU could be used to selectively induce coagulative necrosis of target tissue in animal experiments [13–17]. Numerous patients with primary liver tumors, breast cancers, renal cancers, and uterine myomas have been treated with HIFU since 1997 [18•,19–21].We have successfully treated 96 cases of bone tumors using HIFU technology until
[%page%]
History and rationale of high intensity focused ultrasound treatment of bone tumors
Since an ultrasound beam attenuates sharply in osseous tissues, ultrasonic energy has limited ability to penetrate and accumulate in bone tissue. Furthermore, the acoustic impedance of bone differs greatly from that of the surrounding soft tissue, which leads to strong reflection to the therapeutic ultrasound beams. Therefore, HIFU was not considered as a suitable modality to treat bone diseases. However, Smith et al. [23] found that focused ultrasound beams can target bone tissues and induce necrosis of osteocyte in normal rabbits. In fact, after the bone was destroyed by a lesion, such as an aneurysmal bone cyst, the lesion inside the marrow cavity could be verified by diagnostic ultrasound imaging [24]. Most malignant bone tumors or osteosarcomas are osteolytic and tend to destroy the normal structure of bone. This suggests that ultrasound beams at lower frequencies may penetrate osseous tissues and thus, it may be possible to use focused ultrasound to treat bone tumors. Recent studies have shown that focused ultrasound at frequencies of 1 MHz or lower could create a sharp focal point and induce tissue destruction in rabbit brain after propagation through a segment of human skull [25,26]. We have reported that a HIFU beam at 0.8 MHz operating frequency could induce coagulation necrosis of VX2 neoplastic tissue in the medullary cavity of rabbit tibia [27]. Within the treated region, tissue became completely necrotic encapsulated with a congestion rim (Fig. 1). The margin between the treated and untreated region was quite clear and definitive. In the same study, coagulative necrosis was found at the destroyed cortex bone, as well as in tumor tissue at deep layer of cortex bone. These findings indicated that HIFU might penetrate through the destroyed bone tissue to ablate tumor tissue at deeper layers. Microscopic histologic examination showed coagulative necrosis in the treated region, and the distance between treated and untreated region was of several cells in thickness [28], suggesting that the delivery of HIFU for the management of bone tumors was relatively precise and controllable. In the targeted region, the destruction of endothelium cells of microvessels and thrombosis was readily detected, suggesting that HIFU could potentially prevent hematogenous dissemination of the tumor cells [28].
Another study further demonstrated that the treatable diameters of bone tumor increased with absorption ratio of bone marrow to tumor, acoustic window of surface skin, and diameter of bone, but decreased with muscle depth and specific absorption rate ratio (SARR) of the bone tumor to the surface skin, bone marrow, and bone [29]. In addition, the optimal driving frequency was dependent on the tumor depth, ultrasound absorption of bone marrow, and bone diameter, but was independent on the acoustic window area and SARR ratio under the three SARR criteria [29]. The findings are helpful to make quick assessment on whether the bone tumor is treatable or not in the multilayer tissue conditions, as well as to choose the optimal operating frequency of the ultrasound transducer and the acoustic window on the skin surface once the parameters, such as the tumor size, tumor depth, and diameter of bone, are known.
Figure 1. Coagulative necrosis and congestion rim surrounding ablated tissue.
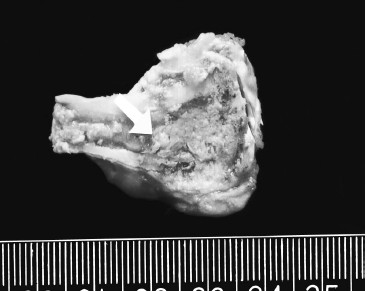
After exposure to high intensity focused ultrasound, targeted tissue became coagulative necrosis
and there was a congestion rim surrounding the ablated tissue. Original photo.
[%page%]
To explore the feasibility and safety of HIFU for managing malignant bone tumors, we conducted a pilot HIFU study on tumor lesions in five patients with osteosarcoma who were undergoing amputation [30]. Clinical symptoms, imaging changes, and functions of vital organs were evaluated before and after HIFU procedure. Samples were retrieved after the patients received amputation procedures and evaluated histologically. Authors found that ECG had no change before, during, and after HIFU procedure, and there were no significant changes in renal function and blood electrolytes before and after HIFU. We did note that the ALP activity (a measure of liver function) increased 3 days after HIFU, but returned to the same or lower pre-HIFU level 1 week later. These preliminary findings indicated that HIFU ablation had no significant influence on the vital organs. Using digital subtraction angiography (DSA), we found that tumor staining in the treated areas disappeared, while small arteries and the blood vessels >
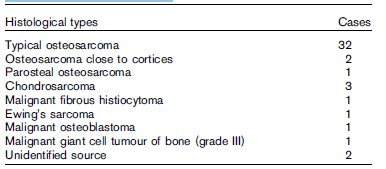
Based on the aforementioned studies, we went on using HIFU procedure on four patients with primary stage II malignant bone tumors, including three chondrosarcoma and one malignant giant cell tumor of bone, and on four patients with breast cancer bone metastases with HIFU ablation alone [31]. Magnetic resonance imaging (MRI) and 99mTc-MDP bone scan before and after HIFU ablation were obtained and compared. We found that all treated regions after HIFU had no intensification and there was an even, thin intensification rim around the region. In 99mTc-MDP bone scan, disappearance of radioactive uptake was found and a radioactive cold region was produced; the treated region covered the entire lesion, suggesting possibly complete inactivation of the tumor foci. Among the eight patients, two chondrosarcoma patients received HIFU treatment twice and another received HIFU just once. After an average follow-up of 23.1 months, no local recurrence was found in any of the cases. These results suggest that HIFU can be used as an effective stand-alone therapy to manage malignant bone tumors. We also conducted further studies using the combination of HIFU and chemotherapy for managing malignant bone tumors. A total of 44 patients with osteosarcomas were treated using HIFU in combination with chemotherapy [32]. Tumor types are included in Table 1. After a mean follow-up time of 17.6 months, the overall survival rate of 44 cases was 84.1%. For the 34 cases of stage IIb, 30 cases continued to survive disease-free, two died of lung and brain metastases, and the other two had local recurrence. Among 10 of the stage IIIb cases, five survived with tumor, one had local recurrence, and five died of lung metastases. The complication occurrence rate was 18.2%, including two cases with secondary infection, three with pathologic fracture, one with epiphysis separation, and two with common peroneal nerve injury. Enneking comprehensive function scoring of the 36 cases was not less than 15 points. It is conceivable that a limb salvage procedure can be carried out to treat malignant bone tumors of limbs using HIFU and chemotherapy because our results demonstrated that HIFU is effective and well tolerated. We expected that HIFU ablation would result in fewer complications and well-preserved limb function because there were no surgical traumas and blood vessels >
Table 1. Histological types of 44 patients with malignant bone tumours who received HIFU.
[%page%]
Figure 2. 99mTc-MDP SPECT before high intensity Figure 3. Fourteen days after high intensity
focused ultrasound treatment. focused ultrasound
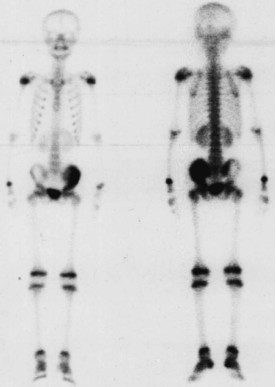
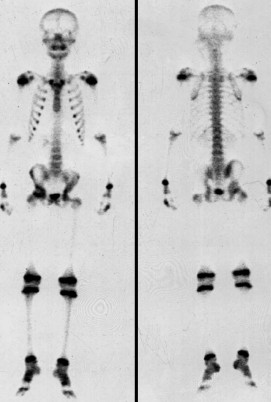
99mTc-MDP SPECT before high intensity focused ultrasound Image obtained 14 days after high intensity focused
treatment demonstrated focal area of increased radioactive uptake ultrasound showed that the tracer uptake of
in a 12-year-old boy with pelvic osteosarcoma, which the treated tumor disappeared completely in the
corresponded to a local abnormality (arrows). Original photo. treated region, indicating absence of viable tumor
cells (arrows). Original photo.
Imaging evaluation post high intensity focused ultrasound ablation
Since HIFU is a noninvasive therapy, it is important to evaluate the therapeutic effectiveness using various imaging techniques. CDFI, X-ray scan, computed tomography (CT) image, DSA, and MRI are usually considered valuable imaging modalities [30,32,34]. It has been reported that CDFI showed a continuous echo enhancement immediately after HIFU, but the enhancement gradually decreased within several weeks. The regions with echo enhancement changes correlated to the tumor areas with extensive necrosis. The tumor volume decreased, or did not change, but the tumor blood supply drastically decreased or disappeared. Using X-ray scan or CTimaging, Zeng et al. [35] found that the tumor volume decreased and calcification within tumors were readily detected. Furthermore, when the treated patients were analyzed by DSA imaging, it was found that the blood accumulation and vascularity of the tumor mass significantly decreased or were undetectable [35,36]. We further analyzed the relation between tumor coagulative necrosis and the post- HIFU DSA and 99mTc-MDP SPECT imaging analyses, and found that coagulative necrosis correlated with the decrease or disappearance of blood accumulation and vascularity in the tumor mass, while 99mTc-MDP SPECT imaging revealed the disappearance of radioactive uptake corresponding to the necrotic tumor region (Figs. 2 and 3). These imaging changes were commonly observed in the HIFU-treated malignant bone tumors [30]. In a follow-up study, it was found that at 3–6 months after HIFU ablation, the HIFU-targeted tumor-bearing bone region regained radioactive uptake on 99mTc-MDP SPECT analysis, suggesting revascularization of inactivated bone areas [35,37]. MRI imaging was able to accurately display the range, dimension, and margin of the ablated bone tumors and, subsequently, inactivated tumor and/or residual/recurrent tumors. After HIFU ablation, T1WI and T2WI were shortened and no intensification was detected after enhanced by Gd-DTPA. The margin between treated and untreated regions was clear with linear intensification, and signals became unified [38•,39•] (Figs. 4 and 5). Thus, 99mTc- MDP SPECTand enhanced MRI imaging should be considered as valid and accurate imaging methods to evaluate the efficacy of HIFU ablation of malignant bone tumors
[%page%]
Figure 4. Contrast-enhanced magnetic resonance image Figure 5. Contrast-enhanced magnetic resonance image
before high intensity focused ultrasound ablation. obtained fourteen days after high intensity focused ultrasound.
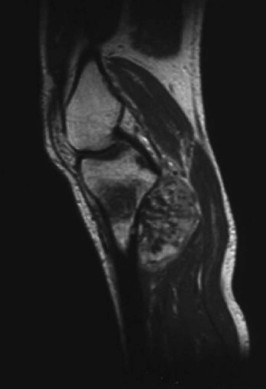
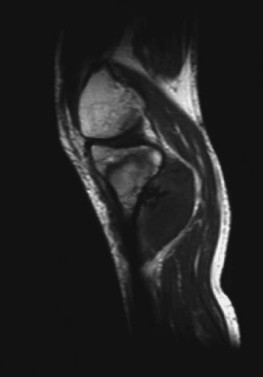
Contrast-enhanced magnetic resonance image before high intensity Contrast-enhanced magnetic resonance image obtained
focused ultrasound ablation showed clear intensification and clear 14 days after high intensity focused ultrasound
vascular perfusion inside the tumor mass (arrow) in a 45-year-old showed absence of intensification in the tumor lesion,
patient with osteosarcoma at the upper right tibia. Adapted with and a thin intensification margin surrounding the
permission [31]. treated lesion (arrow). Adapted with permission [31].
Effect of high intensity focused ultrasound ablation on host immune response
One of the surprising findings is that HIFU ablation is seemingly to improve the functionality of the host immune system, although the mechanisms behind this phenomenon remain to be defined. Animal experiments have demonstrated that, while there were a few HSP70 positive cells in rabbit VX2 bone tumors before HIFU ablation, HSP70 positive cells significantly increased up to 3 weeks after HIFU treatment [40]. Furthermore, HIFU ablation was shown to increase CD25 positive cells in the same rabbit bone tumor model [40]. It has been speculated that the increase of HSP70 and CD25 positivity in malignant bone tumors may facilitate tumor-specific antigen presentation to T lymphocytes, which stimulate the proliferation of T lymphocyte and enhance the anti-tumor immune response. In a clinical trial, 16 patients with solid malignancies, including six patients with osteosarcoma, were treated with HIFU. The peripheral T lymphocytes, B lymphocytes, and natural killer cells (NK) in the peripheral blood were measured by flow cytometry in the patients 1 day before HIFU and 7–10 days after HIFU. There were significant increases in the population of CD4 (+) lymphocytes (P < 0.01) and in the ratio of CD4 (+)/CD8 (+) (P < 0.05) in the cancer patients after HIFU ablation [41]. The elevated levels of CD3 (+) lymphocytes, CD4 (+)/CD8 (+) ratio, CD19 (+) lymphocytes, and cytotoxic NK cells returned to the normal range in most, if not all, HIFU-treated patients [41]. The findings suggest thatHIFU ablation may enhance the host anti-tumor immunity in addition to its local tumor destruction in patients with solid malignant tumors. Similar results were reported from another study [42]. It has also been reported that HIFU ablation may eliminate or significantly reduce potential circulating tumor cells in the patients with solid malignancies [43], suggesting that HIFU treatment per se does not enhance the risk of tumor metastasis. While the long-term therapeutic benefit and molecular mechanism of the HIFU-treatment–elicited host immune response in cancer patients require further investigation, it is conceivable that HIFU ablation may induce massive tumor cell death and/or necrosis, which could lead to the release of tumor antigens to stimulate host anti-tumor immune response. These changes may ultimately enhance the immune function of tumor-bearing patients and improve their prognosis [44].
Conclusion
As a noninvasive technique, extracorporeal HIFU has been shown to effectively induce coagulative necrosis in several types of solid malignant tumors including osteosarcomas. The targeted ablation can be monitored and evaluated using the conventional imaging techniques, such as CDFI, X-ray radiography, CT scan, MRI imaging, and 99mTc-MDP SPECT. Preclinical and clinical trial results have demonstrated that HIFU ablation is a well tolerated and effective treatment for malignant bone tumors. However, further investigation and long-term follow-up studies are needed to validate the therapeutic efficacy, the function status of salvaged limbs, and incidence of complications of HIFU ablation. Nevertheless, HIFU ablation is a novel, promising, and noninvasive tumor treatmentmodality, especially for the cases in which tumors can not be removed surgically.